Despite two COVID-19 vaccines approved, 69% Indians still hesitant to take the COVID-19 vaccine
- ● Percentage of hesitant citizens remains unchanged at 69% from Dec 2020 to Jan 2021
- ● Only 26% Indian parents say they are interested to get Covid vaccine for their children before the start of the school session in April 2021
- ● 61% citizens want the Government to get Pfizer and Moderna to conduct COVID-19 trials for commercial use in India
January 6, 2021, New Delhi: Countries from around the world are gearing up for a largest vaccination drive ever recorded in history to drastically reduce the spread of coronavirus infection. With vaccines developed by Pfizer-BioNTech, Oxford-Astrazeneca and Moderna Inc emerge as frontrunners, there’s a hope that the curve will flatten very soon. However, this is a mammoth task at hand for a country like India keeping in mind its population, infrastructure, supply and distribution, storage, affordability, etc. – notwithstanding the hesitancy of people to take the vaccine. The Drug Controller General of India (DCGI) has given a green signal to Oxford-Astrazeneca’s and Serum Institute of India's Covishield, along with Bharat Biotech's Covaxin last weekend. While Covishield has been authorized for emergency use only, final contours are reportedly being worked out for vaccination of Covishield as it is yet to fulfill additional conditionalities. However, the big question is how many Indians are ready to take the vaccine? The Indian Government has conducted an end-to-end mock drill on the vaccine administration on January 3, 2020, across States and UTs at 286 session sites spread across 125 districts.
Since October 2020, LocalCircles has been collecting responses from citizens to know their approach on taking the COVID-19 vaccine, aimed to understand if the percentage of reluctance or hesitancy has increased, reduced or remains unchanged. 61% of citizens in the October survey expressed hesitancy in getting the vaccine. With vaccine makers – Pfizer and Moderna – announcing success on efficacy results, the aggregate percentage of citizens in India hesitant about the vaccine reduced to 59% in the November survey. The vaccine developed by Oxford-AstraZeneca via Serum Institute of India gave promising hope to India. However, LocalCircles survey instead indicated a dramatic increase in the percentage of citizens to 69% who were hesitant over taking the vaccine. Another independent survey conducted in December by LocalCircles member by Dr Abdul Ghafur, indicated that even majority of the healthcare professionals – or, 55% were hesitant about COVID-19 vaccine. These majority of professionals in the survey maintained that they are worried about side-effects, or not sure about its efficacy. Approximately 60% of these professionals were engaged with a Covid facility.
On the other hand, citizens believe enough information is not available when it comes to vaccine side-effects, efficacy, etc. from trials, which combined with declining case loads in India are top reasons why people are becoming hesitant to take the COVID-19 vaccine. Few experts warn of lack of transparency regarding the approval process, too, with DCGI not taking any questions from media when announcing the approval of Covishield and Covaxin on January 3, 2020 is of concern. Earlier, when Serum was conducting trials for Covishield, a participant who undertook the trial had alleged that the vaccine was causing him serious side-effects, both neurological and psychological. Serum dismissed the claims as “oblique pecuniary motive” maintaining that the participant’s suffering was independent of the vaccine trial he underwent and threatened to seek damages for malicious allegations in excess of INR 100 crores (20 times the damages claimed by the participant). All such issues have led to a level of distrust building amongst citizens. It has been reported that the vaccine Covaxin developed by Bharat Biotech has received the drug regulator’s green signal for administration to children over 12 years. LocalCircles conducted another survey on vaccine hesitancy, change over time, as well as people’s view on Government of India making Pfizer and Moderna vaccines available via commercial channels.
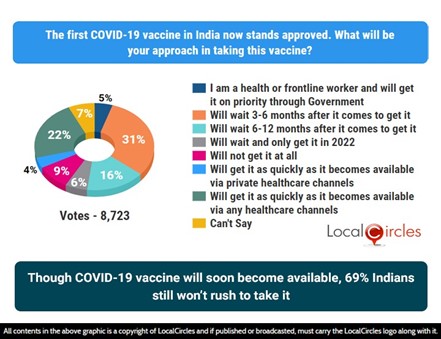
Though COVID-19 vaccine will soon become available, 69% Indians still won’t rush to take it
The survey conducted by LocalCircles between January has concluded that Indians’ hesitancy over COVID-19 vaccines remains unchanged in November and December 2020, with 69% maintaining that they won’t rush to take it. To the question that received 8,723 responses, only 26% of citizens said that they will get it as soon as it becomes available via private or any healthcare channels, while 5% said they are health or frontline workers and will get themselves vaccinated on priority through government channels. The latest survey result indicated that the hesitancy of citizens remains unchanged at 69% unwilling to take it despite two vaccines receiving the regulator’s green signal for vaccination. This percentage remains unchanged with the result published in December 2020. In November and October 2020 surveys, 59% and 61% of citizens were hesitant to take the vaccine.
Through COVID-19 vaccine will soon become available, 69% Indians still won’t rush to take it
Despite multiple vaccines now approved, 69% citizens remain hesitant about taking one immediately

Only 26% Indian parents approve of their children receiving the Covid vaccine if it is made available in April 2021 (before next school session); 56% want to wait atleast till July 2021

Only 26% Indian parents approve of their children receiving COVID-19 vaccine by April 2021
India’s drug regulator has reportedly given the green signal to Bharat Biotech’s Covaxin for administration on children above 12 years old. When LocalCircles asked parents “If the COVID-19 vaccine is made available for school children, would you consider giving it to your child or grandchild?” Only 26% Indian parents approved of their children receiving the COVID-19 vaccine if it is made available by April 2021, or before school session. Further 56% of parents voted “Will wait 3 months or more and then consider based on data or findings'', while 12% said “No”. The survey to the question received 10,468 responses. This is noteworthy because it suggests that a large majority are hesitant at this point to give a Covid vaccine to their children. With 69% parents in a LocalCircles survey saying they want schools to open from April 2021 or after, it is clear that with declining caseloads parents are becoming more comfortable to send children to school and only a small percent i.e. 26% are seeking vaccine safety for their children.
61% of citizens want the Government to get Pfizer and Moderna to conduct COVID-19 trials in India so they can also be available commercially at the earliest
In the race to develop the most effective COVID-19 vaccines, ones developed by Pfizer-BioNTech and Moderna have been more promising than others, with efficacy levels of nearly 95%. Both vaccines require storage temperatures of -70 degree centigrade and -20 degree centigrade respectively. The AstraZeneca-Oxford-SII Covid-19 vaccine can be stored, transported and handled at normal refrigerated conditions (2°C to 8°C) for at least six months. In participants who received two full doses of AstraZeneca-Oxford Covid-19 vaccine at least one month apart, its efficacy was 62 per cent; in participants who received a low dose followed by a full dose, efficacy was 90 per cent.
61% citizens want Government to get Pfizer and Moderna to conduct COVID-19 vaccine trials in India so they can also be available commercially at the earliest

The Pfizer-BioNTech vaccine has already received clearance in the United States, Britain, the European Union and a dozen other countries, while the U.S. government is considering half the dose of Moderna’s COVID-19 vaccine to people in speed vaccinations. In India, per report, Pfizer and Moderna have received several co-productions offers from Indian companies. While the India Government’ negotiations with Moderna have reportedly hit a roadblock, Pfizer is yet to submit details as asked by the drug regulator.
LocalCircles asked citizens, “Should the Government make efforts to get Pfizer and Moderna to conduct COVID-19 vaccine trials in India such that these vaccines can also be made available commercially at the earliest?” In the survey conducted among 9,121 citizens, it was found out that 61% citizens want the Government to conduct the test of these two vaccines in India. As the poll itself suggests that a majority of Indians want the vaccine trials of Pfizer and Moderna to be conducted here in the country, it is important that the Government enables it. There has been an increase in citizens’ posts suggesting that there should be different Covid-19 vaccines to choose from as to speed up the road to recovery from the pandemic.
India is a densely populated country, and the number of coronavirus tally has already crossed the one million mark in the ongoing pandemic. The LocalCircles survey indicates that vaccine hesitancy concerns among citizens have percolated down to the general public, with 69% still hesitant despite two vaccines receiving the regulator’s approval. This is likely to have a negative consequence on India’s effort to control the spread of COVID-19 unless the Central and State Governments, along with pharmaceutical industry work in tandem to keep citizens updated at every step about the vaccine efficacy, side-effects and safety concerns, including adverse events and successes in a transparent manner. There’s also the risk of fake information and it becoming viral is high, with high penetration of social media. Authentic and timely information coming from the authorities and vaccine makers can play a big role in minimizing such risk and converting the hesitant citizens.
Survey Demographics
18,000+ responses were received from citizens residing in 224 districts of India. 69% respondents were men while 31% respondents were women. 51% respondents were from tier 1, 31% from tier 2 and 18% respondents were from tier 3, 4 and rural districts. They survey was conducted via LocalCircles platform and all participants are validated citizens who had to be registered with LocalCircles to participate in this survey. An additional 10,000+ responses were received on the poll on vaccination of children in a related LocalCircles survey.
About LocalCircles
LocalCircles, India’s leading Community Social Media platform enables citizens and small businesses to escalate issues for policy and enforcement interventions and enables Government to make policies that are citizen and small business centric. LocalCircles is also India’s # 1 pollster on issues of governance, public and consumer interest. More about LocalCircles can be found on https://www.localcircles.com
For more queries - media@localcircles.com, +91-8585909866
All content in this report is a copyright of LocalCircles. Any reproduction or redistribution of the graphics or the data therein requires the LocalCircles logo to be carried along with it. In case any violation is observed LocalCircles reserves the right to take legal action.


